In this example water is broken down into its two elements. In order for a substance to conduct electricity it must contain charged particles charge carriers that are sufficiently mobile to move in response to an applied electric field.
What Is Needed For Evaporation To Occur Explain In Terms Of Intermolecular Forces Socratic
The same thing holds true of ionic compounds when melted.

. Would you expect a glass of water to evaporate more quickly on a windy. A gas must be held in a closed container to prevent it from moving out into its surroundings. In the case of ionic compounds in water solutions the ions themselves serve this function.
The result is a chemical change because the starting and ending molecules are different. For instance when an electric current is passed through water H2O it can be broken down into hydrogen and oxygen or H2 O2. Solid liquid and gas.
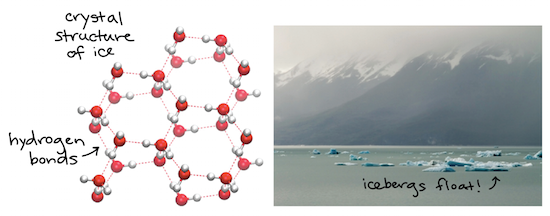
C Particles in a gas are separated by distances that are considerably larger than the size of the particles themselves and they move about freely. When water freezes it becomes hard and less dense but it is still chemically the same. Water is the only known substance on Earth that exists naturally in three states.
Recent reports on the formation of hydrogen peroxide H2O2 in water microdroplets produced via pneumatic spraying or capillary condensation have garnered significant attention. Although most health officials say that soap and water is the best way to keep your hands virus-free when youre not near a sink the experts. To change between these states water must undergo physical changes.
How covalent bonds in water could break under such mild conditions challenges our textbook understanding of physical chemistry and. Youll notice that this chemical reaction needed electricity to happen. Which type of bond must be broken for water to vaporize.
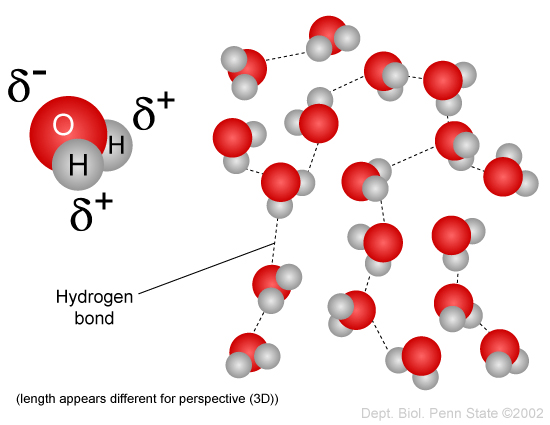
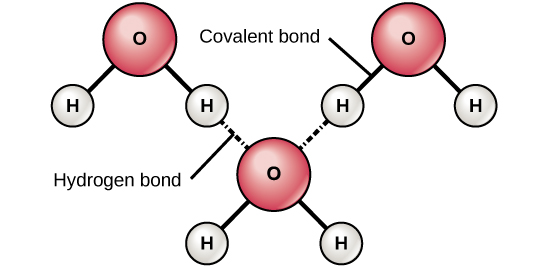
Water for example is made up of two hydrogen atoms and one oxygen atom. Water can flow but it also remains in an open container because of the forces between its molecules. Stable hydrogen bonds keep water molecules of ice farther apart than water molecules of liquid water.

Hydrogen Bonds Make Water Sticky Manoa Hawaii Edu Exploringourfluidearth
Specific Heat Heat Of Vaporization And Density Of Water Article Khan Academy
0 Comments